If not the equation will probably never balance. Chemical reactions and equations Balancing a chemical.
How Do You Balance Double Replacement Reactions Socratic
Correct balancing the equation is a simple task.

. Redox reactions are characterized by the actual or formal transfer of electrons between chemical species most often with one species the reducing agent undergoing oxidation losing electrons while another species the. Predicting and balancing neutralization and precipitation reactions. SINGLE REPLACEMENT REACTIONS DOUBLE REPLACEMENT REACTIONS 1.
Types of Reactions Worksheet Solutions. A 2 NaBr 1 CaOH 2 1 CaBr 2 2 NaOH Type of reaction. Examples of displacement reactions 16.
CuCl 2 H 2 S CuS 2HCl Note that the product is not H 2 Cl 2. This reaction will occur because Mg is more reactive than Cu in the activity series. It is important to recognize that CuCl 2 is made of three ions Cu 2 and two Cl-.
Displacement and double displacement reactions. HBr Type of reaction. Definition and examples of double replacement reactions.
Try balancing these chemical reactions. A and B must be either different metals including H or different halogens. 2 Na H 2 SO 4 Na 2 SO 4.
Single Displacement Reactions aqueous ONLY metals. AlOH3 Type of reaction. In chemistry there is the principle of microscopic reversibility which states that every elementary chemical reaction is reversible an elementary chemical reaction is one that occurs in essentially one step and is not a chemical reaction representing a multistep process.
Examples of Single-Displacement Reactions For our first example well explore the reaction between Mg and CuSO4. Double displacement 3 3 Mg 1 Fe 2O3 2 Fe 3 MgO Type of reaction. MgO Type of.
Types of Reactions Worksheet Solutions Balance the following equations and indicate the type of reaction taking place. Aside from that its the same thing as a double displacement reaction. NaOH HClO 4 NaClO 4 H 2 O In this question you must recognize that perchlorate ClO 4- and hydroxide OH- are polyatomic ions and will not.
For example if two hydrogen molecules collide with an oxygen molecule to produce two water. A list of the seven diatomic elements plus two friends Reaction types and product prediction in a PDF file. The general form of a double-replacement also called double-displacement reaction is.
A s BX aq AX aq B s. A single displacement reaction involves a product where one chemical compound from the reactant side exchanges to the product. Double displacement b 2 NH 3 1 H 2SO 4 1 NH 4 2SO 4 Type of reaction.
1 3 NaBr 1 H3PO 4 1 Na 3PO 4 3 HBr Type of reaction. Here you will learn more about chemical reactions acids bases salts carbon compounds metals non-metals and periodic classification. Chemical reactions and equations.
In chemistry chemical reactions are frequently written as an equation using chemical symbols. As you can see the H and B switched places which is where the water came from. In a single-displacement reaction one element displaces another element in a compound.
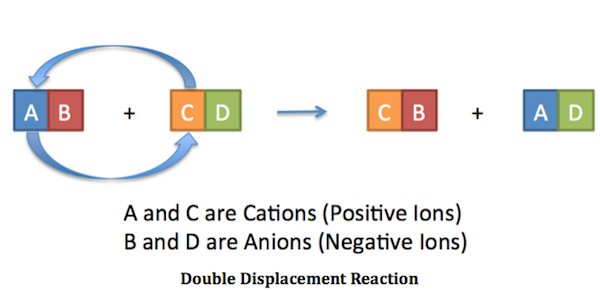
A double-replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds. Double displacement 2 3 CaOH 2 1 Al 2SO 43 3 CaSO 4 2 AlOH 3 Type of reaction. Writing molecular and net ionic equations.
P 4 3NaOH 3H 2 O 3NaH 2 PO 2 PH 3. The reactants are displayed on the left side of the equation and the products are shown on the right with the separation of either a single or double arrow that signifies the direction of the reaction. Combustion d 3 Pb 2 H 3PO.
A B-C A-C B. These reactions can either come in the form of single replacement where one element in a compound is replaced by another one or in the form of a double replacement where an element in each of the two different reactants gets replaced. CeAB ceCD rightarrow ceAD ceCB.
Six balancing by groups problems. A displacement reaction can be single or double. HA BOH BA H₂O.
Chemistry is part of everything in our lives. In this type of reaction we can find a hydrogen displacement and sometimes rarely occurring reactions involving oxygen displacement. Single displacement 4 1.
Balance the following reactions and indicate which of the six types of chemical reaction are being represented. These reactions are also called precipitation reactions as an insoluble substance is formed which is known as a precipitate. Synthesis c 4 C 5H 9O 27 O 2 20 CO 2 18 H 2O Type of reaction.
The general equation for a single-displacement reaction is. In a double displacement reaction two sets of exchanges. Displacement reactions with an oxidation state change.
There are five types of chemical reactions and equations that represent them. Double displacement reactions Reactions in which there is an exchange of ions between the reactants are called double displacement reactions. In any case acid-base reactions are pretty much the same thing as double displacement reactions except that water is one of the things thats made.
Balance the following equations and indicate the type of reaction taking place. Single displacement double displacement synthesis decomposition or. In these reactions there is never a change in oxidation state in other words the charges stay the same.
The reactions in which a single reactant is oxidized and reduced is known as Disproportionation reactions. Types of Reactions Balancing and Predicting Products Balance the following equations and also tell what type they are. Sixteen balance redox equations by sight.
EXAMPLES AgNO3 NaCl --- AgCl NaNO3 Silver nitrate sodium chloride silver chloride sodium nitrate. Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of atoms are changed. Chemists have devised an Activity Series.

Double Replacement Reaction Practice Problems Examples Youtube

Double Replacement Double Displacement Reaction

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com
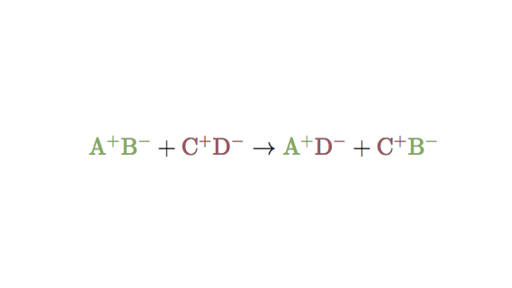
Double Replacement Reactions Double Displacement Article Khan Academy

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Introduction To Double Replacement Reactions Youtube

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

0 comments
Post a Comment